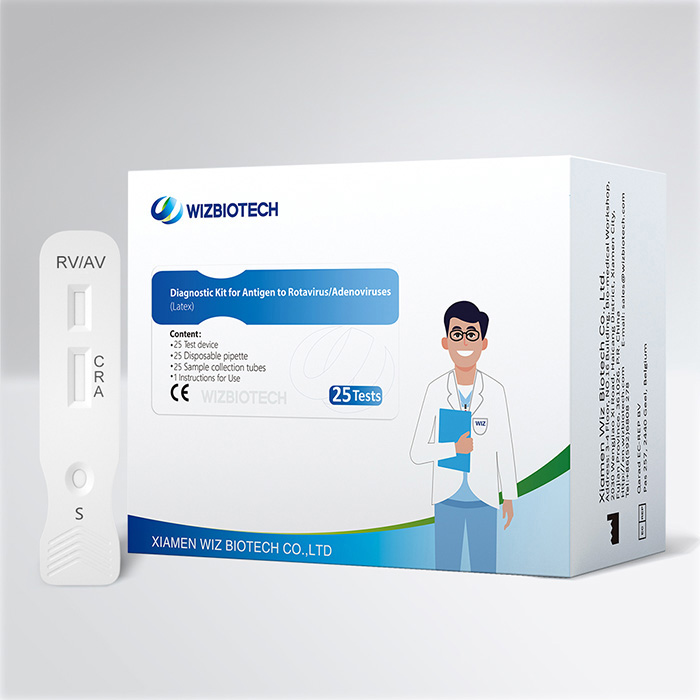
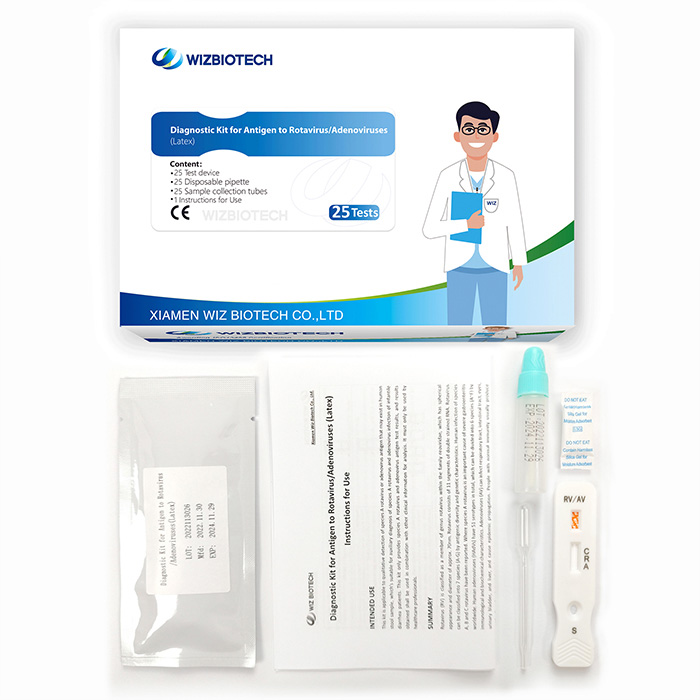
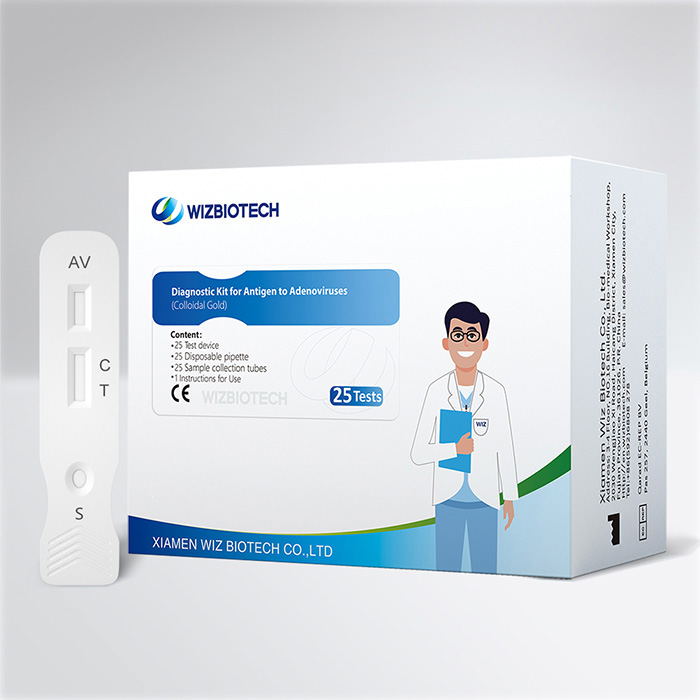
Prueba combinada de diarrea infantil Prueba de látex en heces para detectar antígenos de rotavirus y adenovirus

- Wizbiotech
- QUÉ, UKCA
- Porcelana
El kit de prueba de rota adenovirus se utiliza para la detección cualitativa de antígenos de rotavirus o adenovirus de la especie A que puedan existir en muestras de heces humanas. Los resultados se obtienen en tan solo 10 a 15 minutos, lo que ayuda a los proveedores de atención médica a diagnosticar la infección por rotavirus y adenovirus de la especie A de manera más temprana.
Descripción
La gastroenteritis infecciosa aguda es una enfermedad común en todo el mundo. La mayoría de los casos son causados por patógenos virales. La infección por rotavirus suele presentarse con vómitos agudos seguidos de varios días de diarrea, dolor abdominal tipo cólico, anorexia y fiebre baja. Los bebés y los niños pequeños que presentan deshidratación grave tienen más probabilidades de contraer una infección por rotavirus que por otros patógenos virales que causan gastroenteritis.
El adenovirus es un virus común que puede causar una variedad de resfriado o gripe-infecciones similares. Los adenovirus pueden afectar a personas de todas las edades, pero son más comunes en niños menores de 5 años.
Beneficios
Muy sencillo de utilizar:El kit de prueba de adenovirus rota proporciona todo lo que necesita. Apenas necesita herramientas adicionales para realizar la prueba.
Resultado rápido:El resultado de la prueba se puede obtener en 10 minutos.
Conveniente:Esta es una prueba combinada, puede obtener 2 resultados de prueba al mismo tiempo.
Especificaciones del producto
Método | Látex |
Tipo de muestra | Heces |
| Es hora de obtener resultados | 10~15 minutos |
| Almacenamiento | 2~30 ℃/36~86℉ |
Duración | 24 meses a partir de la fecha de fabricación |
| Tamaño del kit | 1/5/20/25 pruebas |
※ Consulte el prospecto del producto para obtener información adicional sobre el producto.
Rendimiento del producto
| Rotavirus de la especie AResultados de WIZ | Resultado de la prueba del reactivo de referencia | Tasa de coincidencia positiva: 99,36 % (IC del 95 %: 96,48 % ~ 99,89 %) Tasa de coincidencia negativa: 100,00% (IC del 95%: 97,25%~100,00%) Tasa de coincidencia total: 99,66 % (IC del 95 %: 98,09 % ~ 99,94 %) | ||
| Positivo | Negativo | Total | ||
| Positivo | 156 | 0 | 156 | |
| Negativo | 1 | 136 | 137 | |
| Total | 157 | 136 | 293 | |
Adenovirus Resultados de WIZ | Resultado de la prueba del reactivo de referencia | Tasa de coincidencia positiva: 98,54 % (IC del 95 %: 94,83 % ~ 99,60 %) Tasa de coincidencia negativa: 100,00% (IC del 95%: 97,60%~100,00%) Tasa de coincidencia total: 99,32 % (IC del 95 %: 97,55 % ~ 99,81 %) | ||
| Positivo | Negativo | Total | ||
| Positivo | 135 | 0 | 135 | |
| Negativo | 2 | 156 | 158 | |
| Total | 137 | 156 | 293 | |
Aplicaciones
Laboratorio de Urgencias para Pacientes Ambulatorios
Departamentos Clínicos
Hospital comunitario
Departamentos de laboratorio
Centro de Gestión de la Salud
Clínica
Certificaciones